How To Install A Hydrogen Fuel Cell In Your Car

Hydrogen as fuel
Hydrogen equally a fuel for fuel cell electric vehicles
The transport sector is the most challenging with respect to transitioning to a 100% renewable society. Information technology accounts for 14 % of global greenhouse gas emissions amongst economic sectors [ane]. Therefore, to achieve our goal of a fully renewable energy organisation, in that location must be a primal focus focus on transportion.
Hydrogen has many applications, and many people see information technology as the clean fuel of the futurity when it is generated from h2o and returns to it oxidized. Hydrogen-powered fuel cells are increasingly seen as promising pollution-free sources of energy and are at present being used in cars and buses. Furthermore, hydrogen is used in the chemical manufacture to produce ammonia for agricultural fertilizer (the Haber Bosch procedure) and cyclohexane and methanol, which are intermediates in the production of plastics and pharmaceuticals [ii]. Methanol is now besides used as a fuel for transportation applications. This article will focus on hydrogen every bit a fuel for fuel cell electric vehicles (FCEV s).
Properties of hydrogen
Gaseous hydrogen has some outstanding specifications compared to other fuel types, equally tin can exist seen in table one.
| Petrol | Methane | Propane | Hydrogen | |
|---|---|---|---|---|
| Lower explosion limit (%, air) | 15 | 5 | 2,1 | iv |
| Upper explosion limit (%, air) | viii | 15 | 9.5 | 75.6 |
| Wink point ͦC | -twenty | -188 | -104 | -270.8 |
| Lowest ignition energy mJ | 0.8 | 0.3 | 0.25 | 0.017 |
| Density (20 ͦC, ane bar) | 0.vii-0.78 kg/l | 0.718 kg/m3 | 2.01 kg/m3 | 0.089 kg/m3 |
| Boiling indicate ͦC | 30-215 | -161.5 | -42 | -252.7 |
| Critical temperature ͦC | -82.v | 96.6 | -239.iii | |
| Critical force per unit area bar | 45 | 42.ii | 13 | |
| Diffusion coefficient cm2/s | 0.16 | 0.12 | 0.61 |
Tabular array one: Fuel specifications [3].
Equally can exist seen in Table 1, hydrogen has a very wide flammability range (lower and upper explosion limit) compared to other fuels, at between 4% and 75%. The optimal combustion condition is a 29% hydrogen-to-air volume ratio. Detection sensors are almost always installed in hydrogen systems to quickly identify any leak and minimize the potential for undetected flames.
Equally mentioned above, hydrogen is the smallest known molecule. It has a low viscosity, which is why it is prone to leakage. In a confined space, leaking hydrogen can accrue and reach flammable concentrations. Any gas other than oxygen is an asphyxiator in sufficient concentrations. In a closed environment, leaks of any size are a business organization, as hydrogen is impossible for human senses to detect and can ignite in a wide range of concentrations in air. However, proper ventilation and the use of detection sensors tin mitigate these hazards.
Hydrogen has the smallest ignition energy, much lower than that required for other common fuels. This ways that minor sparks can easily ignite it.
Hydrogen has loftier free energy content by weight (density) but not by volume, which is a challenge for storage. In order to store sufficient quantities of hydrogen gas, it is compressed and stored at high pressures. As can be seen in Table 1, the critical pressure for gaseous hydrogen is 13 bar. For comparing, hydrogen is compressed to 350-700 bar in storage tanks in FCEVs. For safety, hydrogen tanks are equipped with pressure relief devices that prevent the force per unit area in the tanks from becoming too high [4].
The easiest way to decrease the volume of a gas, at constant temperatures, is to increase its pressure level. Thus, at 700 bar, hydrogen has a density of 42 kg/chiliadiii, compared to 0.089 kg/k3 under normal pressure level and temperature atmospheric condition. At this force per unit area, 5 kg of hydrogen can be stored in a 125 liter tank [5].
As tin can exist seen in Figure ane, the density of hydrogen highly depends on the temperature and force per unit area.
Effigy i: Hydrogen density at different temperatures and pressures. [5]
Due to its weight, hydrogen has a high diffusion rate, which results in rapid dispersion. This means that if a hydrogen cloud comes into contact with an ignition source in an open space with no confinement, flames will propagate through a flammable hydrogen-air cloud at several meters per second, and even more rapidly if the cloud is above ambient temperature [four].
Hydrogen tin can, nevertheless, exist used as safely as other common fuels when simple guidelines are followed. This volition exist dealt with in the sub-section: Standards and regulations.
The application of hydrogen in the ship sector
Hydrogen can exist used in 3 different means in relation to transportation:
- Fuel prison cell-electrical vehicles
- Hydrogen combustion engines
- Hydrogen-marsh gas-mixtures for combustion engines
This paper volition focus on FCEVs. At that place are two main reasons why FCEVs are superior to electric vehicles (EVs): i) shorter refueling times; and ii) longer ranges [6]. FCEV refueling times are only a few minutes, while an EV needs several hours fully recharge. FCEVs are more efficient than conventional internal combustion engines vehicles and produce no tailpipe emissions, only emitting water vapor and warm air. FCEVs utilise a propulsion system similar to that of electric vehicles, where free energy stored as hydrogen is converted into electricity past the fuel prison cell. Furthermore, they are equipped with avant-garde technologies to increase efficiency, such as regenerative braking systems that capture the energy lost during braking and store information technology in a bombardment [7].
Today, nigh car manufacturers accept opted for a solution that consists of storing hydrogen in gaseous form at loftier pressure. This enables the storage of enough hydrogen to let a FCEVs to travel betwixt 500 and 600 km between refuelings [eight].
Hydrogen fuel applied science has undergone a huge improvement in the terminal few years. In fact, in that location is significantly more energy and explosive potential in a gasoline fuel tank than in a hydrogen fuel jail cell tank. Furthermore, diverse sensors are emplaced on hydrogen-fuel cell vehicles to manage possible leaks, such as [9]:
- Alarms
- Seal valves
- Seal fuel lines
An overview of the differences between gasoline/petrol cars, EVs and FCEVs can be seen in the table below.
| Car type | Gasoline/petrol | EV | FCEV |
|---|---|---|---|
| Charging time (full charge) | ≈ 5 min. | Several hours | ≈ 5 min. [vii] |
| Efficiency (well-to-wheel) [10] | ≈ 13 % | ≈ 73 % | ≈ 22 % |
| Range (km) | Up to ≈ 1200 [eleven] | ≈ 200-500 [12] | ≈ 600 |
| Pollution | Tailpipe emissions from tailpipe. | No tailpipe emissions and the environmental effects depend on power production in the energy system. | No tailpipe emissions. |
| Gravimetric energy density (MJ/kg) [13] | ≈ 35 | ≈ 0-1 | ≈ half-dozen |
| Production cost [xiv] | 1,30 EUR/50 | Depends highly on the location [15] | seven EUR/Kg |
| Consumption per 100 km | 7.04 l/100 km [14] | 17-twenty kWh/100 km [16] | 0.84 kg/100 km [xiv] |
| Cost per km (EUR/100 km) | 9.152 | 0.238-6.02 [17] | 6.65 |
Tabular array 2: Comparison of different vehicle types.
The combining of brusk refueling times, high driving ranges and toll-effectivenessmake FCEVs the about optimal solution.
Hydrogen purity
Hydrogen purity or quality is a term to describe the lack of impurities in hydrogen equally a fuel gas. The purity requirement varies with the awarding. A hydrogen internal combustion engine can tolerate low hydrogen purity, whereas a hydrogen fuel cell requires high hydrogen purity to prevent catalyst poisoning. Impurities in hydrogen (even in the ppm and ppb range) have a severe effect on the performance of fuel cells. Thus, it is crucial to be able to discover any impurities before a fuel is used. An international standard (ISO 14687-2:2012) has been published to specify the impurities and levels that must be identified, as is shown in Tabular array iii.
| Species | Maximum Concentration (µmol/mol)(ppm) |
|---|---|
| Water | 5 |
| Total hydrocarbons | 2 |
| Oxygen | 5 |
| Helium | 300 |
| Nitrogen/Argon | 100 |
| Carbon Dioxide | 2 |
| Carbon Monoxide | 0.2 |
| Total Sulphur compounds | 0.004 |
| Formaldehyde | 0.01 |
| Formic acid | 0.2 |
| Ammonia | 0.1 |
| Total halogenated compounds | 0.05 |
| Maximum particulates concentration | 1 mh/kg |
Table iii: Impurities and levels that must exist identified for hydrogen to be a viable fuel in FCEVs [eighteen].
Hydrogen storage
Hydrogen tin can be stored physically as either a gas or liquid. It typically requires high-pressure tanks (350-700 bar tank pressure). Some other possibility is the chemical storage of hydrogen, whereby it is stored on the surface of solids (by adsorption) or within solids (by absorption). The automotive application utilizes the physical storage of hydrogen [19].
- Compressed gas
- Cold/Cryo-compressed
The storage tanks in cars must withstand high pressures and be able to shop hydrogen without whatever leakage. Several car manufacturers today employ compressed hydrogen tanks in their cars, which are capable of 350 and 700 bars, depending on the automotive blazon (lite duty/heavy duty).
As mentioned above, at that place are ii main varieties of hydrogen fuel tanks. The nigh common hydrogen fuel tank for cars, trucks, buses and other vehicles is the compressed hydrogen gas tank. Virtually hydrogen fueling stations currently manipulate compressed hydrogen and only a few bear cryogenic liquid hydrogen. This is considering almost all car manufacturers take chosen to fuel their cars with compressed hydrogen gas. BMW is 1 exception, with their dual fuel "Hydrogen 7 automobile" that uses cryogenic hydrogen and gasoline.
One disadvantage of cryogenic hydrogen tanks in recent years has been that they can take boil-off issues. This means that the liquid hydrogen volition, over time, find its way out of the tank and evaporate. This is only the case when the machine is left alone in one place for a couple of weeks, e.g., an airport. Furthermore, information technology is necessary to store cryogenic liquid hydrogen at a temperature below negative 253 degrees Celsius to maintain its liquidity. This demands a technologically advanced freezer arrangement.
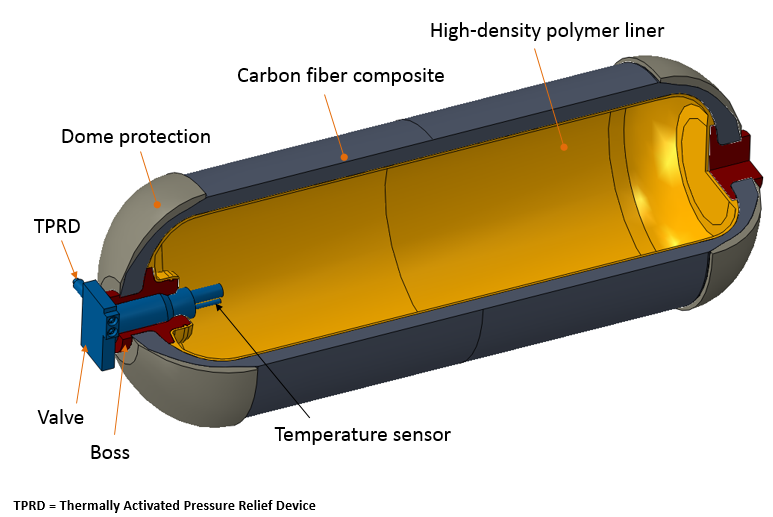
Compressed hydrogen fuel tanks are now made of carbon fiber composites or carbon fiber and metal alloys and composites. The inner line of the tank is a high-molecular weight polymer that serves as a hydrogen gas permeation barrier. The outer shell is placed on the tank for bear upon and damage resistance. A pressure regulator and an in-tank gas temperature sensor are located in the tank's interior in club to monitor the pressure and temperature during the gas-filling process.
Compressed hydrogen gas tanks tin can have different interiors.
- Open infinite
- Metallic hydride engineering
When the hydrogen is stored in the porous metal hydride material, the gas is released past adding a small amount of heat to the tank. The disadvantage of this is that metallic hydrides are generally very heavy, which will cutting down the range per liter of fuel in the vehicle.
The goal is to detect a better way to store hydrogen that is not as plush as metal hydrides or related methods under development. Hydrogen tanks must exist lighter, hold more volume and toll less than they presently do [nineteen].
Several studies take been conducted on fabric-based hydrogen storage to farther improve storage potential. These studies have investigated metallic hydride, chemical hydrogen storage and sorbent materials [21]. Scientists and researchers are currently working on this issue and, as with many other engineering-driven challenges, the future will most probable hold a multifariousness of viable solutions.
Nevertheless, however, today'due south hydrogen fuel tanks are safe due to several safety measures and requirements related to ATEX (European directives for controlling explosive atmospheres) approval requirements.
Standards and regulations
Hydrogen has a few unique properties that require special consideration, as described in the first section, Properties of Hydrogen. The aforementioned considerations apply as with any fuel; rubber handling depends on knowledge of its particular physical, chemic and thermal backdrop and consideration of safe ways to accommodate these. Hydrogen, when handled with knowledge, is a condom fuel.
To ensure that hydrogen is handled properly, the International Arrangement for Standardization (ISO) is developing international prophylactic standards; the Canadian Hydrogen Installation Lawmaking (Chic), for instance, defines the requirements applicable to the installation of hydrogen equipment while the Gild of Automotive Engineers (SAE) defines standards whereby the principal emphasis is placed on the transportation industry. Several standards for hydrogen applications have also been published during the terminal few years [22]:
Hydrogen production:
| Designation | Titlel |
|---|---|
| IThen/DIS 1487 | Hydrogen fuel quality – Product specification |
| ISO/DIS 22734 | Hydrogen generators using water electrolysis process – Industrial, commercial, and residential applications |
| ISO 14687-1:1999 | Hydrogen fuel – Product specification – Part i: All applications except proton exchange membrane (PEM) fuel cell for road vehicles |
| ISO 14687-2:2012 | Hydrogen fuel – Product specification – Part 2: Proton exchange membrane (PEM) fuel jail cell applications in road vehicles |
| ISO 14687-3:2014 | Hydrogen fuel – Product specification – Function 3: Proton exchange membrane (PEM) fuel prison cell application in stationary appliances |
| ISO 16110-1:2007 | Hydrogen generators using fuel processing technologies – Function 1: Safety |
| ISO 16110-two:2010 | Hydrogen generators using fuel processing technologies – Part 2: Test methods for operation |
| ISO/TS 19883:2017 | |
| ISO 22734-1:2008 | Hydrogen generators using h2o electrolysis process – Office i: Industrial and commercial applications |
| ISO 22734-2:2011 | Hydrogen generators using water electrolysis process – Part 2: Residential applications |
Storage and transport:
There are international standards being developed specifically for both the stationary and portable storage of hydrogen, which is critical for ensuring condom in the hydrogen industry.
| Designation | Title |
|---|---|
| ISO 19884 (under evolution) | Gaseous hydrogen – Cylinders and tubes for stationary storage |
| ISO 16111 | Transportable gas storage devices – Hydrogen absorbed in reversible metal hybride |
Safety:
Safety is a disquisitional factor to be considered and is vital for satisfying customs expectations and furthermore, to ensuring workforce and ecology health and prophylactic.
| Designation | Title |
|---|---|
| ISO/TR 15916 | Basic considerations for the safe of hydrogen systems |
| ISO 26142 | Hydrogen detection apparatus – Stationary applications |
Transportation:
Many standards exist for transportation utilization.
| Designation | Title |
|---|---|
| ISO/DIS 17268 (under development) | Gaseous hydrogen state vehicle refueling connectedness devices |
| ISO/DIS 19881 (under development) | Gaseous hydrogen – Land vehicle fuel containers |
| ISO/DIS 19882 (nether evolution) | Gaseous hydrogen – Thermally activated pressure level relief devices for compressed hydrogen vehicle fuel containers |
| ISO/TSO 19880-1 | Gaseous hydrogen – Fueling stations – Function 1: General requirements |
| ISO/TSO 19880-ii | Gaseous hydrogen – Fueling stations – Function 2: Dispenser |
| ISO/TSO 19880-3 | Gaseous hydrogen – Fueling stations – Part 3: Valves |
| ISO/TSO 19880-5 | Gaseous hydrogen – Fueling stations – Part 5: Fueling station hoses |
| ISO/TSO 19880-6 | Gaseous hydrogen – Fueling stations – Part half-dozen: Fittings |
| ISO/TSO 19880-eight | Gaseous hydrogen – Fueling stations – Part 8: Fuel quality control |
| ISO 13984:1999 | Liquid hydrogen – Country vehicle fueling system interface |
| ISO 13985:2006 | Liquid hydrogen – Land vehicle fuel tanks |
| ISO/TS 15869:2009 | Gaseous hydrogen and hydrogen blends – State vehicle fuel tanks |
| ISO 17268:2012 | Gaseous hydrogen land vehicle refueling connection devices |
| ISO/TS 19880-1:2016 | Gaseous hydrogen – Fueling stations – Office 1: General requirements |
| SAE J2600: 2022-08 | Compressed Hydrogen Surface Vehicle Fuelling Connexion Devices |
| SAE J2601: 2022-07 | Standard, Fueling Protocols for Light Duty Gaseous Hydrogen Surface Vehicles |
| SAE J2799: 2022-04 | Standard, Hydrogen Surface Vehicle to Station Communications Hardware and Software |
The ISO is currently developing new standards relating to hydrogen applications. Furthermore, companies that manufacture hydrogen and fuel cell products and build hydrogen fueling stations use many features that continue to be validated through safety tests. Hydrogen has been safely produced, stored, transported and used in large amounts in industrial applications [23] [24].
Summary
Benefits of hydrogen every bit a fuel for fuel jail cell electrical vehicles:
| + No tailpipe pollution |
| + Not-toxic, generated from h2o and returning to water when oxidized |
| + Range and refueling time is comparable to ICEs |
| + fourteen times lighter than air, ascent and disperses rapidly |
| + High energy content by mass |
Drawbacks of hydrogen every bit a fuel for fuel prison cell electric vehicles:
| - Depression viscosity – difficulties in storing hydrogen |
| - Highly explosive and dangerous in closed environments |
| - Pressurized gaseous fuel requires special engines and infrastructure |
| - Low energy content by book |
References
| [i] | EPA, "Greenhouse gas emissions," [Online]. Bachelor: www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data. [Accessed 24 January 2022]. |
| [two] | "Hydrogen," Periodic Table, [Online]. Bachelor: world wide web.rsc.org/periodic-table/element/1/hydrogen. [Accessed 14 February 2022]. |
| [3] | M. Jahn, Wasserstoff als Antrieb, Stuttgart: Regierungspräsidium Stuttgart, 2022. |
| [iv] | H. Tools, "Hydrogen compared with other fuels," Hydrogen Tools, [Online]. Available: h2tools.org/bestpractices/hydrogen-compared-other-fuels. [Accessed v February 2022]. |
| [five] | H. Tools, "Hydrogen Density at different temperatures and pressures.," Hydrogen Tool , [Online]. Available: h2tools.org/hyarc/hydrogen-data/hydrogen-density-different-temperatures-and-pressures. [Accessed 14 Feb 2022]. |
| [6] | A. Thompson, "Where are all the hydrogen cars?," [Online]. Available: www.popularmechanics.com/cars/hybrid-electric/a22688627/hydrogen-fuel-prison cell-cars/. [Accessed thirty January 2022]. |
| [vii] | U. D. o. Energy, "Culling Fuels Data Ceneter," [Online]. Bachelor: afdc.free energy.gov/fuels/hydrogen_basics.html. [Accessed thirty January 2022]. |
| [8] | A. Liquide, "Storing Hydrogen," Air Liquide, 2022. [Online]. Bachelor: energies.airliquide.com/resource-planet-hydrogen/how-hydrogen-stored. [Accessed 5 February 2022]. |
| [9] | T. Southward. Monica, "Are Hydrogen Cell Vehicles Prophylactic?," Toyota Santa Monica, 22 dec 2022. [Online]. Available: www.toyotasantamonica.com/are-hydrogen-prison cell-vehicles-safe/. [Accessed 5 February 2022]. |
| [10] | M. Kane, "Efficiency compared: Bombardment-Electric 73 %, Hyrdogen 22 %, Water ice 13 %," InsideEVs, 2022. [Online]. Available: insideevs.com/efficiency-compared-battery-electric-73-hydrogen-22-ice-thirteen/. [Accessed thirty January 2022]. |
| [xi] | B. Wong, "Peak 10 Vehicles with the longest Driving Range," cars.com, 15 December 2022. [Online]. Bachelor: www.cars.com/manufactures/top-10-vehicles-with-the-longest-driving-range-1420698377103/. [Accessed 14 February 2022]. |
| [12] | Autorader, "Here are the 10 Electrical Vehicles with the longest ranges," Autorader, April 2022. [Online]. Available: world wide web.autotrader.com/best-cars/here-are-10-electric-vehicles-longest-ranges-263793. [Accessed thirteen February 2022]. |
| [xiii] | P. east. a. 2009, "ResearchGate," 2009. [Online]. Available: www.researchgate.cyberspace/effigy/Fuel-energy-densities-cyberspace-systems-volumetric-and-gravimetric-free energy-densities-for_fig1_44691024. [Accessed 30 Jan 2022]. |
| [xiv] | A. K. G. M. J.-P. P. Anna Creti, "A toll benefit analysis of fuel cell electrical vehicles," HAL -archives ouverts, 2022. |
| [fifteen] | e. Southward. Explained, "Electricity price statistics," 2022/2018. [Online]. Bachelor: ec.europa.eu/eurostat/statistics-explained/index.php/Electricity_price_statistics. [Accessed 29 January 2022]. |
| [xvi] | puschevs, "Electric cars: range and efficiency comparing," Pushevs, 2022. [Online]. Available: pushevs.com/2016/11/23/electrical-cars-range-efficiency-comparison/. [Accessed 25 January 2022]. |
| [17] | E. southward. Explained, "Electricity price statistics," Eurostat, 2022/2018. [Online]. Available: ec.europa.eu/eurostat/statistics-explained/index.php/Electricity_price_statistics. [Accessed 25 January 2022]. |
| [18] | A. B. a. A. M. Grand. Downey, "Hydrogen purity Analysis for Fuel Cell Vehicles," NPL (National Physical Laboratory), [Online]. Available: world wide web.scribd.com/document/186290821/Hydrogen-Purity-Analysis-for-Fuel-Cell-Vehicles. [Accessed 14 February 2022]. |
| [19] | G. Kantola, "Hydrogen Fuel Tanks," Hydrogen cars now , 2022. [Online]. Available: world wide web.hydrogencarsnow.com/alphabetize.php/hydrogen-fuel-tanks/. [Accessed seven Feb 2022]. |
| [20] | Energy.Gov, "Physical Hydrogen Storage," Part of Energy Efficiency & Renewable Energy, [Online]. Available: www.energy.gov/eere/fuelcells/concrete-hydrogen-storage. [Accessed fourteen February 2022]. |
| [21] | Energy.Gov, "Materials-based Hydrogen Storage," Office of Free energy Efficiency and Renewable Free energy, [Online]. Available: www.free energy.gov/eere/fuelcells/materials-based-hydrogen-storage. [Accessed v Feb 2022]. |
| [22] | H. T. Standards, "Hydrogen Technology Standards, Word Paper," Standards Commonwealth of australia, 17 Oktober 2022. [Online]. Available: www.standards.org.au/getmedia/2d89a05c-9dd0-4878-90f8-d1c228306d5b/D-1368-Hydrogen-word-newspaper.pdf.aspx. [Accessed fourteen februar 2022]. |
| [23] | CHFCA, "How safe is Hydrogen," CHFCA, [Online]. Available: www.chfca.ca/education-middle/hydrogen-safety/. [Accessed 5 February 2022]. |
| [24] | I. O. f. Standardization, "Standards catalogue," ISO, [Online]. Available: www.iso.org/commission/54560/x/catalogue/p/0/u/i/west/0/d/0. [Accessed seven February 2022]. |
Broadcasting

Source: https://www.ieafuelcell.com/index.php?id=33
Posted by: fieldsthonind1980.blogspot.com


0 Response to "How To Install A Hydrogen Fuel Cell In Your Car"
Post a Comment